Properties and States Of Matter
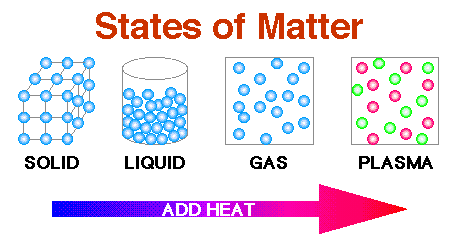 Matter can be classified into 1. Solid 2. Liquid
3. Gas
Matter can be classified into 1. Solid 2. Liquid
3. Gas
States Of Matter Properties
No.
|
States
Of Matter
|
Volume
|
Shape
|
1.
|
Solid
|
Fixed
|
Fixed
|
2.
|
Liquid
|
Fixed
|
Changed
|
3.
|
Gas
|
Changed
|
Changed
|
Solid to
Liquid = Melting
Solid to Gas = Sublimating
Liquid to Solid = Freezing
Liquid to Gas = Evaporating
Gas to Solid = Sublimating
Gas to Liquid = Condensating
Particles Of Matter
Matter
consist of tiny part
called Particles. Particle cannot be seen by naked eyes.
The Properties of particles are :
1. Particles are not idle, they always
vibrate.
2. There are attractive force between
particles.
3. There are free space between
particles which are known as pores.
The Particles Characteristics Of
Matter
No
|
Particle
Characteristics
|
Solid
|
Liquid
|
Gas
|
1.
|
Particle
Movement
|
Rigid
|
Rapid
|
Very
Rapid
|
2.
|
Particle
Position
|
Dense
|
Loose
|
Very
Loose
|
3.
|
Attractive
Forced
|
Strong
|
Weak
|
Very
Weak
|
4.
|
Free
Space Between Particles
|
Slight
|
Spacious
|
Very
Spacious
|
Cohesion and Adhesion
Those force
may be classified as Cohesion and Adhesion. Cohesion is
the tedency of certain similar particles to cling together due to attractive
force. Adhesion is tedency of certain dissimilar particles to cling
together due to attractive force.
1. Concave Meniscus and Convex Meniscus
The water’s surface is curving upward and its wets the tube wall because
the attractive force among the waters particles is smaller than the attractive
force between the water’s particles and the tube wall (Cohesion smaller than
Adhesion).
The Mercury’s face is curving downward and doesn’t wets the tube wall
because the attractive force among the mercury’s particles is greater than the
attractive force between mercury’s particles ang the tube wall (Cohesion is
greater than Adhesion).
The upward curve of liquid’s surface is called concave meniscus, while
the downward curve is called convex meniscus.
Capilarity
Capilarity is the rising of liquid up or down in a capilarity tube.
Density
1. Density as a Characteristics of
Matter
Density is defined matematically as : p = M/V
With : P = Density (Kg/m3)
M = Object mass (KG or G)
V
= Object’s volume (m3 or cm3)
